Research Experience
University of Arizona Tissue Optics Laboratory
Duke University Center for Global Women's Health Technologies
University of Arizona Tissue Optics Laboratory

I am currently developing an endoscopic imaging system for early detection of cancer
SiPhox Health
Duke University Center for Global Women's Health Technologies
University of Arizona Tissue Optics Laboratory

I developed diagnostic assays for at-home health tracking
Duke University Center for Global Women's Health Technologies
Duke University Center for Global Women's Health Technologies
University of Arizona Maximizing Access to Research Careers (MARC) Program

I investigated the anti-metastatic effects of a novel, local tumor ablation method in triple-negative breast cancer
University of Arizona Maximizing Access to Research Careers (MARC) Program
University of Arizona Maximizing Access to Research Careers (MARC) Program

I helped develop a novel biosensor for detecting cancer cells in the blood at the point-of-care
Massachusetts Institute of Technology Summer Research Program (MSRP)

Using 3D microfluidic models, I investigated the interactions between T cells and human blood vessels in the presence of cancer
Howard Hughes Medical Institute Exceptional Research Opportunities (EXROP) Program

I optimized a portable assay for detecting substandard medicines in developing countries
Danish Institute for Study Abroad (DIS) Science Research Practicum (SRP)

I studied the effects of aging and exercise on skeletal muscle and how muscle fibers regenerate after injury
UCSD Medical Scientist Training Program Summer Undergraduate Research Fellowship (SURF)

I tested a hydrogel as a drug delivery method for treating cardiovascular disease
Interdisciplinary Consortium on Advanced Motion Performance (iCAMP) Internship
Interdisciplinary Consortium on Advanced Motion Performance (iCAMP) Internship

I ran clinical trials for a virtual reality-based exercise program to help HIV patients improve their walking and balance abilities
University of Arizona Tissue Optics Laboratory

Dates
August 2023 - Present
Position
Research Assistant (PhD Student)
Mentor
Jennifer Barton, PhD, Director of the BIO5 Institute, Professor of Biomedical Engineering and Optical Sciences
Host University
University of Arizona
Project Title
Developing an endoscopic imaging system for early detection of cancer using optical coherence tomography (OCT), doppler OCT (DOCT), and optical coherence elastography (OCE)
Methods and Responsibilities
- Designing and constructing endoscope, including optical system and piezoelectric scanning mechanism
- Conducting ex vivo preliminary studies with a benchtop OCT system for proof-of-concept
Publications
Gonzales, Alana G., Caitlin Ruhland, Graham Spicer, Stephen Mead, Massimiliano Di Pietro, Ashraf Sanduka, Photini F. S. Rice, Ryan H. W. Mitstifer, Sarah E. Bohndiek, Travis W. Sawyer, and Jennifer K. Barton. "Optical coherence tomography and elastography for ex vivo visualization of early gastric cancer," manuscript submitted to Journal of Biomedical Optics as of July 2025.
Rocha, Andrew D., Dominique Galvez, Alana Gonzales, Eduardo Gonzalez, Matthias Schlich, David Vega, and Jennifer K. Barton (2025). “Lens design strategies for miniature scanning fiber endoscopes,” Optical Engineering, vol. 64(9), 090801.
Conference Proceedings
Gonzales, Alana, Ruyuan Dong, Ryan Walton Mitstifer, Caitlin Ruhland, Travis Sawyer, Ghassan Mouneimne, and Jennifer K. Barton (2025). “Optical coherence tomography and elastography for the visualization of architecture and stiffness differences in soft- and stiff-conditioned murine mammary tumors,” Proc. SPIE 13306, Advanced Biomedical and Clinical Diagnostic and Surgical Guidance Systems XXIII, 133060E.
Rocha, Andrew D., Matthias Schlich, David Vega, Jennifer K. Barton, Alana Gonzales, Eduardo Gonzalez, and Dominique Galvez (2025). "Finite element and optical analysis of a miniature forward-scanning fiber endoscope", Proc. SPIE PC13334, Endoscopic Microscopy XX, PC1333403.
SiPhox Health

Dates
June 2022 - August 2023
Position
Associate Research Scientist
Company Website
Project Title
Silicon Photonics System for Low-Cost Rapid Quantification of Biomarkers in Blood
Abstract
We introduce a low-cost swept-source optical reader and disposable microfluidic cartridge system for multiplexed point-of-care and at-home diagnostics using photonic microresonators. In a triple-blind study of high-sensitivity C-Reactive Protein (hsCRP) quantification in blood plasma, our system demonstrated good agreement (R>0.9) with gold-standard lab measurement techniques.
Methods and Responsibilities
- Developed point-of-care and at-home diagnostic assays with robust photonic chip technology
- Planned and carried out experiments, analyzed results, and made decisions to progress research
Conference Proceedings
Kyle Preston, Guojun Chen, Cole Chapman, Sarat Gundavarapu, Ebrahim Aljohani, Armando Paredes Arroyo, Chi-Chen Lin, Alexander Vinitsky, Alana Gonzales, Ze Yin, Michael Dubrovsky, and Diedrik Vermeulen (2024). “Silicon Photonics-Powered Biosensing for Rapid Quantification of Blood Biomarkers,” Frontiers in Optics + Laser Science 2024 (FiO, LS), Technical Digest Series (Optica Publishing Group, 2024), paper FTu1D.1.
Aljohani, Ebrahim, Sarat Gundavarapu, Cole A. Chapman, Armando Paredes Arroyo, Guojun Chen, Chi-Chen Lin, Andrea Romig, Kyle Preston, Alexander Vinitsky, Alana Gonzales, Eric Hsu, Jordan Cobb, Yulia Rybakova, Michael Dubrovsky, Diedrik Vermeulen (2023). “Silicon Photonics System for Low-Cost Rapid Quantification of Biomarkers in Blood,” 2023 IEEE Silicon Photonics Conference (SiPhotonics), 1-2.
Duke Center for Global Women's Health Technologies

Dates
July 2020 - May 2022
Position
Graduate Researcher
Host University
Duke University
Mentor
Nirmala Ramanujam, PhD, Robert W. Carr Professor of Engineering and Professor of Cancer Pharmacology and Global Health
Project Title
Targeting Tumor Acidosis and Regulatory T Cells Unmasks Anti-Metastatic Potential of Local Tumor Ablation in Triple-Negative Breast Cancer
Abstract
Triple-negative breast cancer (TNBC) is an immunologically heterogenous disease that lacks clinically actionable targets and is more likely to progress to metastatic disease than other types of breast cancer. Tumor ablation has been used to increase response rates to checkpoint inhibitors, which remain low for TNBC patients. We hypothesized that tumor ablation could produce an anti-tumor response without using checkpoint inhibitors if immunosuppression (i.e., Tregs, tumor acidosis) was subdued. Tumors were primed with sodium bicarbonate (200 mM p.o.) to reduce tumor acidosis and low-dose cyclophosphamide (100–200 mg/kg i.p.) to deplete regulatory T cells, as has been shown independently in previous studies. A novel injectable ablative was then used to necrose the tumor, release tumor antigens, and initiate an immune event that could create an abscopal effect. This combination of bicarbonate, cyclophosphamide, and ablation, called “BiCyclA”, was tested in three syngeneic models of TNBC: E0771 (C57BL/6), 67NR (BALB/c), and 4T1-Luc (BALB/c). In E0771 and 67NR, BiCyclA therapy significantly reduced tumor growth and cured 5/7 and 6/10 mice 50 days after treatment respectively. In the metastatic 4T1-Luc tumors, for which surgery and checkpoint inhibitors fail, BiCyclA cured 5/10 mice of primary tumors and lung metastases. Notably, CD4+ and CD8+ T cells were found to be crucial for the anti-metastatic response, and cured mice were able to resist tumor rechallenge, suggesting production of immune memory. Reduction of tumor acidity and regulatory T cells with ablation is a simple yet effective therapy for local and systemic tumor control with broad applicability as it is not limited by expensive supplies.
Methods and Responsibilities
- Conducted longitudinal studies in mice to assess survival, tumor growth, and anti-tumor immune response following breast tumor polymer-assisted ethanol ablation
- Quantified tumor growth over time with in vivo bioluminescence imaging
Publication
Nief, Corrine, Alana Gonzales, Erika Chelales, Júlia Sroda Agudogo, Brian T. Crouch, Smita Nair, Nirmala Ramanujam (2022). “Targeting tumor acidosis and regulatory T cells unmasks anti-metastatic potential of local tumor ablation in triple-negative breast cancer,” International Journal of Molecular Sciences, vol. 23, 8479.
Conference Proceedings
Agudogo, Júlia, Corrine Nief, Alana Gonzales, Erika Chelales, Rebecca Previs, Jenna Mueller, Brian Crouch, and Nimmi Ramanujam (2022). “A novel point-of-care ethyl cellulose ethanol ablative therapy for the enhancement of paclitaxel in local recurrent cervical cancer treatment (308.5),” Gynecologic Oncology, vol. 166, supplement 1, S162.
Nief, Corrine A., Júlia Sroda Agudogo, Alana Gonzales, Rebecca A. Previs, Smita K Nair, and Nimmi Ramanujam (2021). “Resetting the tumor microenvironment to favor anti-tumor immunity after local ablation,” Journal of Clinical Oncology, vol. 39, issue 15_suppl, 2561.
Agudogo, Júlia Sroda, Corrine Nief, Erika Chelales, Alana Gonzales, Jenna Mueller, Brian Crouch, Rebecca A. Previs, and Nimmi Ramanujam (2021). “A novel treatment for recurrent localized cervical cancer using point-of-care ethyl cellulose ethanol ablation with concurrent cytotoxic therapy,” Journal of Clinical Oncology, vol. 39, issue 15_suppl, e17507.
Maximizing Access to Research Careers Program

Dates
Summer 2018 - May 2020
Position
MARC Trainee
Host University
University of Arizona
Mentor
Jeong-Yeol Yoon, PhD, Professor of Biomedical Engineering
Project Title
Smartphone Based On-Chip Fluorescence Imaging and Capillary Flow Velocity Measurement for Detecting ROR1+ Cancer Cells from Buffy Coat Blood Samples on Dual-Layer Paper Microfluidic Chip
Abstract
Diagnosis of hematological cancer requires complete white blood cell count, followed by flow cytometry with multiple markers, and cytology. It requires substantial time and specialized training. A dual-layer paper microfluidic chip was developed as a quicker, low-cost, and field-deployable alternative to detect ROR1+ (receptor tyrosine-like orphan receptor one) cancer cells from the undiluted and untreated buffy coat blood samples. The first capture layer consisted of a GF/D glass fiber substrate, preloaded with cancer specific anti-ROR1 conjugated fluorescent particles to its center for cancer cell capture and direct smartphone fluorescence imaging. The second flow layer was comprised of a grade 1 cellulose chromatography paper with wax-printed four channels for wicking and capillary flow-based detection. The flow velocity was used as measure of antigen concentration in the buffy coat sample. In this manner, intact cells and their antigens were separated and independently analyzed by both imaging and flow velocity analyses. A custom-made smartphone-based fluorescence microscope and automated image processing and particle counter software were developed to enumerate particles on paper, with the limit of detection of 1 cell/μL. Flow velocity analysis showed even greater sensitivity, with the limit of detection of 0.1 cells/μL in the first 6 s of assay. Comparison with capillary flow model revealed great alignment with experimental data and greater correlation to viscosity than interfacial tension. Our proposed device is able to capture and on-chip image ROR1+ cancer cells within a complex sample matrix (buffy coat) while simultaneously quantifying cell concentration in a point-of-care manner.
Methods and Responsibilities
- Designed and built an imaging apparatus to improve the quality of smartphone fluorescence imaging using SolidWorks and 3D printing
- Created a graphical user interface for simple, automated processing of fluorescence images in MATLAB
Publications
Ulep, Tiffany-Heather, Ryan Zenhausern, Alana Gonzales, David S. Knoff, Paula A. Lengerke Diaz, Januario E. Castro & Jeong-Yeol Yoon (2020). “Smartphone based on-chip fluorescence imaging and capillary flow velocity measurement for detecting ROR1+ cancer cells from buffy coat blood samples on dual-layer paper microfluidic chip,” Biosensors and Bioelectronics, vol. 153, 112042.
Chung, Soo, Lane E. Breshears, Alana Gonzales, Christian M. Jennings, Christina M. Morrison, Walter Q. Betancourt, Kelly A. Reynolds & Jeong-Yeol Yoon (2021). “Norovirus detection in water samples at the level of single virus copies per microliter using a smartphone-based fluorescence microscope,” Nature Protocols, vol. 16, 1452-1475.
MIT Summer Research Program

Dates
Summer 2019
Position
MSRP Intern
Host University
Massachusetts Institute of Technology
Mentor
Roger Kamm, PhD, Professor of Biological and Mechanical Engineering
Project Title
Modeling T Cell and Vascular Interactions In Vitro
Abstract
Immunotherapy drugs have become a widespread cancer treatment. Researchers are developing methods to predict patient-specific responses, but these have not examined the influence of the vascular endothelium in immune cell migration to tumors. We aim to model this interaction using in vitro systems by evaluating T cell migration and adhesion to human endothelial cells (ECs). Initial 2D experiments used conditioned media from four cancer cell lines to provide cytokine stimulus. Porous membranes were used to quantify T cell migration in response to a cytokine gradient, while adhesion was measured by treating EC monolayers with conditioned media prior to incubation with T cells. There was a significant difference in T cell adhesion across the four cell lines (p=1.8×10-5) and in T cell migration in response to cytokine gradients (p=2.5×10-3). Results showed a nearly 3-fold increase in T cell adhesion to ECs (p=3×10-5) and a 3-fold increase in T cell migration (p=0.0017) in response to MDA-MB-231 conditioned media compared to the negative controls. Therefore, this model demonstrates that cytokine stimulus affects T cell migration and adhesion to EC monolayers. Future experiments aim to examine migration and adhesion profiles in more detail using vascularized 3D microfluidic devices to model the tumor microenvironment.
Methods and Responsibilities
- Designed two experimental setups to model T cell adhesion and migration relative to human endothelial cells using a Transwell migration assay, 2D cell culture, and fluorescence microscopy
- Wrote scripts in ImageJ and RStudio to analyze microscopy data
MSRP 2019 Video
Learn more about the unique MSRP experience and my incredible fellow interns.
HHMI Exceptional Research Opportunities Program

Dates
Summer 2017
Position
EXROP Fellow
Host University
Boston University
Mentor
Muhammad Zaman, PhD, Professor of Biomedical Engineering
Project Title
Optimizing a Dihydroartemisinin Assay for the Detection of Substandard Medicines
Abstract
Globally, about 10 to 30 percent of pharmaceuticals are of poor quality, a significant portion of which are classified as substandard, displaying reduced bioavailability due to lapses in manufacturing and handling. This project is expanding the pharmaceutical testing library for PharmaChk, a portable, robust, and user-friendly tool for quantification of active pharmaceutical ingredient (API), to address the need to screen for substandard medications in the field. To improve the accuracy and efficiency of an assay for dihydroartemisinin (DHA), a common antimalarial drug, sample preparation and signal acquisition settings on PharmaChk were explored. Quantification of DHA from tablet samples on PharmaChk was demonstrated, and results were verified using a Molecular Diagnostics SpectraMax M5 spectrophotometer. There was no significant difference between DHA quantification using a spectrophotometer and quantification on the instrument. These results support our ability to quantify the API of DHA tablets on the PharmaChk platform. Adding DHA to PharmaChk’s testing library contributes to the overall goal of addressing the need to screen for substandard medications in the field. Future studies optimizing additional assays can further expand the breadth of PharmaChk’s quantification capabilities.
Methods and Responsibilities
- Performed luminescence measurements using spectrophotometry and a portable microfluidic device
- Improved the accuracy and precision of measurements by troubleshooting pharmaceutical sample preparation and signal acquisition parameters
Blog Post
During my time in the Zaman Lab, I practiced scientific communication by writing a blog post about my project.
DIS Science Research Practicum
Dates
Spring 2017
Position
Student Researcher
Host Institution
Institute of Sports Medicine, Bispebjerg Hospital, Copenhagen, Denmark
Mentor
Abigail Mackey, PhD
Project Title
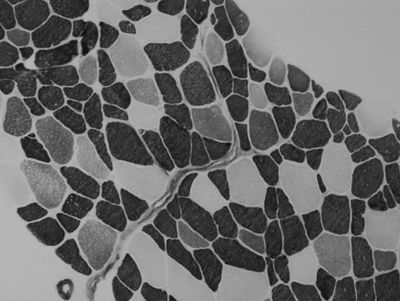
Determining Skeletal Muscle Fiber Type in Regenerating Muscle after Injury Induced by Electrical Stimulation of the Vastus Lateralis
Abstract
The purpose of this study was to characterize injured skeletal muscle fibers regenerating post-injury. Based on discoveries in previous research studying the mechanisms of muscle regeneration, we wanted to look further into the interactions between the cells involved in this process. One study done by Abigail Mackey and colleagues (2011) observed that after induced injury, multiple muscle fiber membranes on serial sections of human gastrocnemius muscle biopsies encroached into one fiber, fusing what was observed as two or more fibers in other locations on the biopsy. We hypothesized that only those fibers which are type II myosin are effected by the electrical stimulation, exhibiting signs of regeneration indicated by fusing and the presence of embryonic and/or neonatal myosin. However, a second hypothesis stated that if a fiber expresses immature myosin, ATPase stains cannot be relied on to determine fiber type. To test the hypotheses, we analyzed serial sections from skeletal muscle biopsies collected following electrically induced eccentric contractions in the vastus lateralis muscle. Methods included imaging of the prepared slides and characterization of the fibers by measuring the size and length of regenerating fibers and determining their myosin type. The results, which showed that none of the type I fibers and most of the type II fibers expressed neonatal myosin, led us to conclude that only type II fibers were affected by the electrical stimulation. We were also able to confirm that the ATPase stain analysis could accurately determine fiber type for those positive for neonatal myosin, but not those expressing embryonic myosin.
Methods and Responsibilities
- Quantified amounts of different fiber types in injured skeletal muscle tissue using fluorescence microscopy
Publication
Højfeldt, Grith, Trent Sorenson, Alana Gonzales, Michael Kjaer, Jesper L Andersen, Abigail L Mackey (2023). “Fusion of myofibre branches is a physiological feature of healthy human skeletal muscle regeneration,” Skeletal Muscle, vol. 13, 13.
MSTP Summer Undergraduate Research Fellowship
Dates
Summer 2016
Position
Summer Fellow
Host University
University of California, San Diego
Mentor
Karen Christman, PhD, Professor of Bioengineering
Project Title
Efficacy of an Extracellular Matrix-Based Hydrogel as a Delivery Method for MicroRNAs
Abstract
MicroRNAs (miRNAs) have demonstrated therapeutic potential due to their role in post-transcriptional gene regulation. However, complications in delivering miRNAs due to rapid degradation by RNases, uncontrolled diffusion, and poor cell uptake have hindered the success of these therapeutics. This study examines the efficacy of an extracellular matrix (ECM)-based hydrogel as a delivery method for miRNAs for the purpose of developing potential treatments for peripheral artery disease (PAD) and myocardial infarction (MI). We hypothesized that the ECM hydrogels would yield a slow release of miRNAs while preventing degradation. For the purpose of our study, we focused on delivering antimir and antagomir oligonucleotides, which inhibit the function of other miRNAs. Skeletal muscle and myocardial ECM hydrogels were produced by decellularizing porcine-derived tissue with detergent, followed by lyophilization, milling, and pepsin digestion. Antimir and antagomir conjugated with a Cy3 dye were mixed with the ECM hydrogels, and phosphate-buffered saline (PBS) supernatant was collected every 24 hours to quantify the amount of antimir or antagomir released via fluorescence readings with a plate reader. Samples from the release study were examined for signs of degradation using gel electrophoresis. Overall, the results of this study will improve the therapeutic efficacy of miRNAs for later in vivo applications.
Methods and Responsibilities
- Fabricated the ECM-based hydrogel by decellularizing porcine tissue with appropriate reagents
- Measured the rate of release of microRNAs from the hydrogel using spectrophotometry
Interdisciplinary Consortium on Advanced Motion Performance

Dates
Summer 2015
Position
Research Assistant
Host University
University of Arizona
Mentors
David Armstrong, PhD, DPM and Bijan Najafi, PhD
Project Title
Gait and Balance Assessment and Training Program for Patients with or At Risk for Peripheral Neuropathy
Project Description
HIV patients and cancer patients undergoing neurotoxic chemotherapy often experience symptoms of peripheral neuropathy. These include pain, tingling, and numbness in the hands and feet as well as weakness and trouble with walking and balance. Patients with peripheral neuropathy commonly experience falls and often require the use of walking assist devices such as canes or wheelchairs. This project tested the effectiveness of a virtual reality-based exercise program for improving gait and balance in HIV and cancer patients affected by peripheral neuropathy. Using wearable sensors, patients completed walking and balance tasks such as a virtual obstacle course, for which the patient steps over virtual objects they see on the screen.
Methods and Responsibilities
- Directed patients through virtual reality-based exercises
- Collected exercise data using MATLAB and wearable motion sensors
- Obtained patient demographics and health history using IRB-approved surveys